How Dolomite regulate the soil pH?
Dolomite is a type of limestone that contains both calcium carbonate (CaCO3) and magnesium carbonate (MgCO3). It is commonly used in agriculture to regulate soil pH, particularly in acidic soils. The process by which dolomite regulates soil pH involves several chemical reactions:
Neutralization: The primary mechanism by which dolomite regulates soil pH is through neutralization of acidity. When dolomite is applied to acidic soils, the carbonate ions (CO3^2-) in dolomite react with hydrogen ions (H+) in the soil solution. This reaction results in the formation of water (H2O) and carbon dioxide (CO2), effectively neutralizing the acidity in the soil:
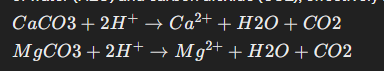
Calcium and Magnesium Availability: Additionally, the calcium (Ca^2+) and magnesium (Mg^2+) ions released during the dissolution of dolomite contribute to soil fertility. These essential nutrients improve soil structure, enhance nutrient availability, and support healthy plant growth.
It's important to note that while dolomite can effectively raise soil pH and provide calcium and magnesium, its use should be carefully managed. Over-application of dolomite can lead to excessive levels of calcium and magnesium in the soil, which may negatively impact soil structure and nutrient balance. Additionally, dolomite is not suitable for use in soils that are already alkaline, as it can raise the pH to levels that are unfavorable for plant growth. Therefore, dolomite application should be based on soil testing and recommendations from agricultural experts to ensure optimal results without adverse effects on soil and plant health.






